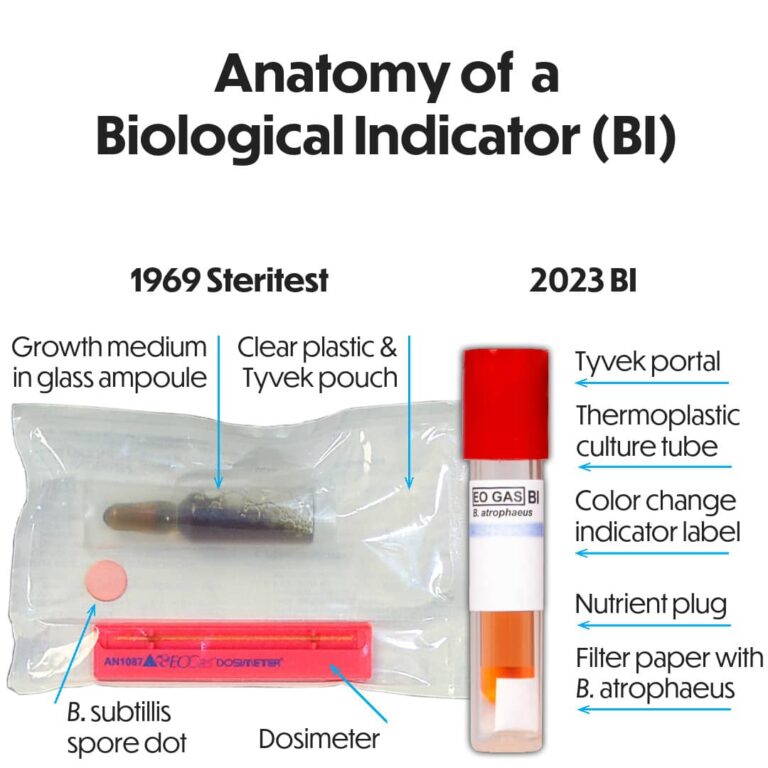
Why Is Ethylene Oxide Used For Sterilization Of Syringes?
More than half of all medical devices in the U.S. are sterilized using ethylene oxide (EO) gas. Instruments made with plastic, like syringes, surgical gloves, and IV tubes, cannot withstand the high temperatures used by other sterilization methods to achieve terminal sterility. Ethylene oxide, however, achieves terminal sterility at low temperatures and with zero damage….