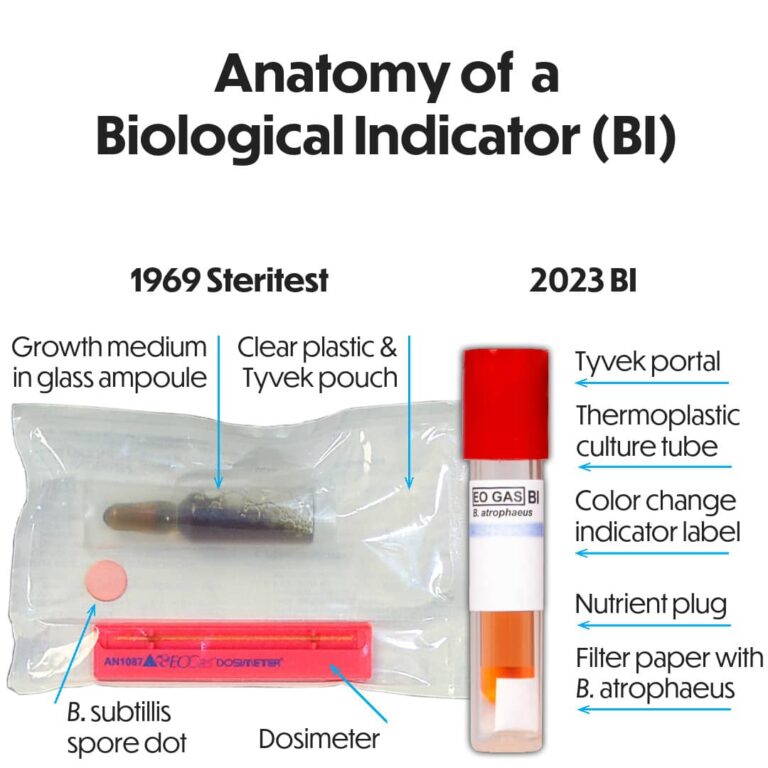
Biological Indicator – An Industry-Sparking Innovation
Andersen Sterilizers founder, H.W. Andersen, M.D., designed and patented the first self-contained Biological Indicator (BI) in 1969. Long after his patent expired, his invention has become an industry in and of itself.